When in solution (dissolved in water) the molecules of an acid or a base dissociate (disunite) into a mix of component parts and the full molecule. The components are electrically charged + or – ions. Ions are unstable and ‘want’ to bond chemically to become stable. In strong acids and bases many ions are present, making them reactive and dangerous.
pH – WHAT IS IT?
The pH is the measure of the amount of free H+ ions in a solution. The measure of pH ranges from 0 at the acid end to 14 the base end (alkaline). Each full division is ten times stronger than the previous one (a logarithmic scale). The mid-point is 7 and represents neutral, distilled water.
pH MEASUREMENT
The most popular industrial method to measure pH is with an electrode that generates a voltage directly related to the H+ concentration. Figure No. 1 is a drawing of a pH probe.
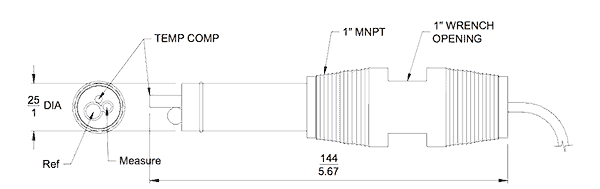
The measuring electrode is sintered glass or ceramic and contained within it there is a solution of known pH concentration. When immersed in the process chemical the electrical properties of the measuring electrode change.
A reference electrode is used to compare the effect of the chemical under test with a known and stable pH solution contained within it. An electrical circuit between the measuring electrode and the reference electrode is maintained through a porous, wetted plug. It forms a ‘liquid junction’ between the reference electrode’s outer surface and the internal chambers containing the reference liquid.
A third sensing element is required to provide temperature compensation to maintain reading accuracy.
FACTORS AFFECTING pH MEASUREMENT
The process variables to be considered include –
• the retention or hold-up time needed for chemical interactions within the process to come to completion,
• the transfer time needed for the dosing system to add the neutralising agent (either acid or base) when required,
• the measurement lag time between the change in pH and the probe’s detection of the change,
• the process mixing rate and amount of agitation available to properly mix the chemical being measured so a pH measurement reflects the bulk chemical’s pH,
• the chemical and physical properties of the process which can affect the measurement quality, such as corrosion, erosion, sediment, caking, etc on the probe.
The pH probe requirements to provide accuracy include –
- time for pH to stabilise without rapid changes,
- stable temperature without extremes,
- clean electrode surfaces,
- full probe contact with the liquid being measured,
- sufficient conductivity through the process chemical,
- no poisoning or drying of the reference electrode.
OPERATIONS ISSUES
Chemical compatibility is critical and the appropriate plastics, elastomers and metals need to be selected for the service. Hot acid and hot base vapours will work their way into the probe’s internals unless it is suitably protected.
The probe has to be easy it get to for maintenance. The internal solutions within the electrode of the probe require periodic replenishment. If the probe becomes coated in caked-on residue or coagulating agents it will need repetitive cleaning. Be careful when removing the probe if build-up is encrusted around it. It must be removed straight and not tilted because the post-type glass electrode will crack if bent.
The probe must not intrude into a thoroughfare or work area as it will be knocked and broken. If mounted in a drainage sump that needs cleaning make the probe removable or protect it from impact by shovels and cleaning equipment.
The liquid junction of the reference electrode must always be wet. If it dries out the pH probe is destroyed. In such situations use probes with a wetting spray that regularly squirts water onto the liquid junction to moisten it.
Mike Sondalini – Maintenance Engineer
If you found this interesting, you may like the ebook Bulk Materials Handling Introduction.
Our pH probes need to be replaced too frequently in the wastewater treatment plant. I am researching possible reasons. Can you think of an instance when a water treatment plant would electrostatically damage a probe?